Abstract
Introduction - The recent GALLIUM study showed an advantage of 7% in progression free survival in favor of obinutuzumab (G) vs. rituximab (R) based immunochemotherapies as first line therapy in follicular lymphoma (FL) patients. Yet, the toxicity appears to be increased with obinutuzumab based therapy.
Methods - To assess for toxicity we compared treatment for FL in the era before the approval of obinutuzumab by the Israeli health authorities on 1/1/2018 (rituximab-based therapy) to the era post approval (obinutuzumab-based therapy). This is a multicenter retrospective cohort study comprising five medical centers in Israel. All adult FL patients treated with first line chemo-immunotherapy were included. Comparison of G vs. R based chemo-immunotherapy was performed in order to assess real life toxicity profile between the two anti-CD20 monoclonal antibodies (R- and G-groups). Primary outcome was any infection recorded during the induction and 6 months post induction period. Secondary outcomes included rates of febrile neutropenia, severe infections defined as grade 3-4 infections, infections requiring intravenous (IV) antibiotics, life threatening infections or fatal infections, as well as rates of other non-hematological and hematological toxicities and treatment discontinuation. Response rates at the end of induction therapy were also recorded.
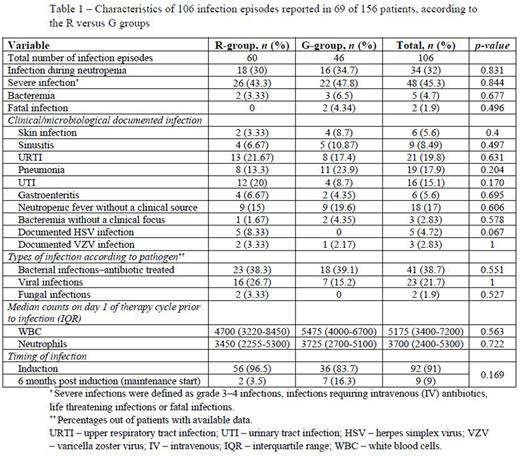
Results - A total of 156 patients, treated between 1/1/2016-1/1/2019, were included in the analysis - 78 patients in each group. Most patients received bendamustine (n= 92, 59%) or CHOP (n=49, 31.4%) as the adjacent chemotherapy regimen. Baseline characteristics of patients were comparable between groups, apart from a higher incidence of diabetes mellitus and hypertension in the R-group. Any infection occurred in 69 patients (44.2%) of the cohort, and a total of 106 infectious episodes were recorded. Patients in the R- and G-groups had similar rates of any infection (44.8% and 43.5%, p=1), severe infections (43.3% vs. 47.8%, p=0.844), febrile neutropenia (15% vs. 19.6%, p=0.606) and treatment discontinuation (14.1% vs. 19.2%). Characteristics of the 106 infection episodes according to the R- versus G- groups are described in Table 1. Most other adverse events recorded, including hematological and non-hematological toxicities, were also comparable between groups, apart from higher rates of nausea and vomiting (30.7% vs. 11.5%, p=0.029) and fever from a non-infectious origin (32% vs. 10%, p=0.032) in the R-group. Overall, no statistically significant difference was evident in adverse events of grades 3 to 5 (hematological and non-hematological) between R and G-based treatments (76.9% vs. 82%, p=0.427) nor in complete remission rates at the end of induction therapy (79.5% vs. 84.6%, p=0.147).
Baseline factors significantly associated with any infection in univariate analysis included female gender, chronic obstructive pulmonary disease, use of GCSF prophylaxis, elevated LDH, beta2 microglobulin and lower levels of hemoglobin before `treatment commencement. None of the parameters was found to be significantly associated with any infection in multivariable analysis, albeit gender and LDH values had a trend towards significance.
Conclusions - In this first and largest real-life study of first line treated patients with follicular lymphoma, we did not observe excess toxicity with G- compared to R- based therapy during the induction and 6 months post induction period.
Disclosures
Berger:Janseen: Honoraria; Neopharm: Honoraria. Nachmias:AbbVie, BMS: Membership on an entity's Board of Directors or advisory committees, Other: lectures. Raanani:Janssen: Speakers Bureau; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Research Funding; BMS: Consultancy. Gafter-Gvili:Medison: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Gurion:Roche: Honoraria; Gilead: Honoraria; Medison Ltd: Honoraria; Takeda: Honoraria; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.